
A Joint and Implant Manufacturer
issued a recall for thousands of plastic inserts used in hip, knee and ankle replacements due to the plastic starting to wear too early leading to implant failure and revision surgery.
The Federal Drug Administration (FDA) classifies this issue as a Class 2 recall because of the risks and damage it can cause. In some cases, a patient may need to have corrective surgery to reverse the damage.
WHY IS THERE A RECALL?
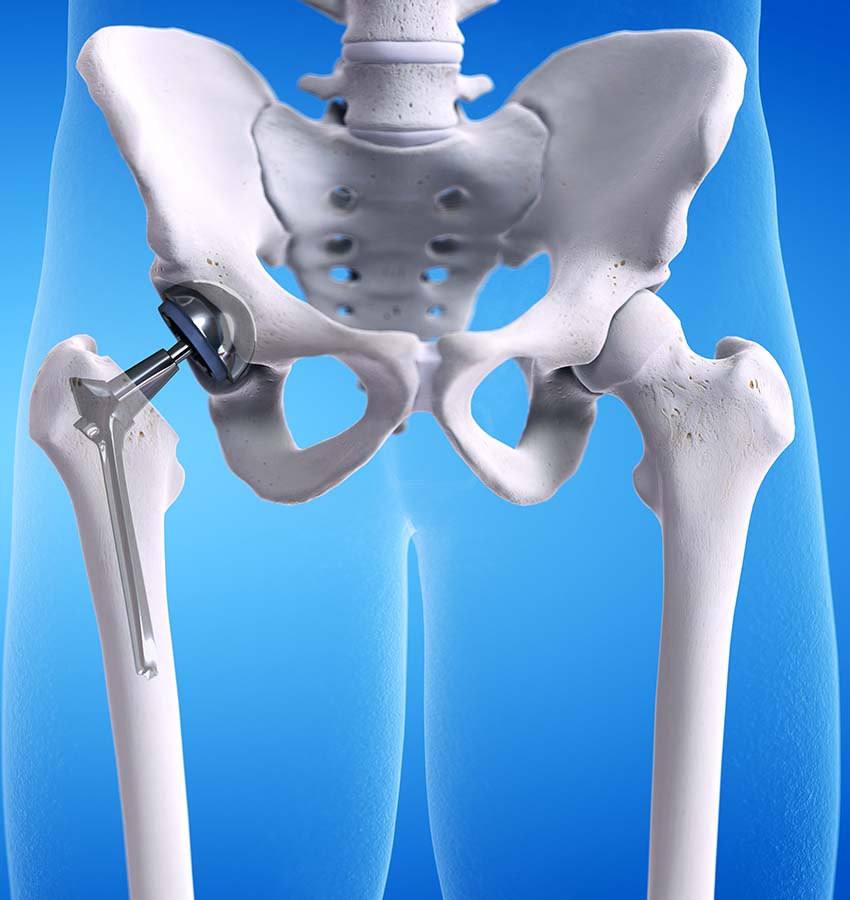
Medical device company Exactech announced that their implant systems made after 2004 had a defect in the vacuum-seal packaging, causing the polyethylene insert component to oxidize and degrade. This packaging defect has caused many implants to prematurely fail forcing many patients to undergo unnecessary corrective surgery.
Did you suffer from serious complications after being implanted with an Exactech knee, ankle, or hip device?
GET A FREE CASE REVIEWWHAT SHOULD I DO IF I HAD EXACTECH HIP SURGERY?
You need help right away, we get it to you in
three simple steps.
1
With comfort and ease fill out our simple, confidential form
2
A U.S. based claims specialist will contact you at a convenient time
3